First of all, the medical plastic injection mold company must understand the dimensional requirements and material requirements of key parts of the product from the client.
1 Material: Polyamide 66 + Semi-aromatic polyamide – 40 % Filler Content
1.1 Regrinding: 0% of the maximum allowable regrinding
1.2 Compounded, Colored, or Other Non-Original Resin Manufacturer Formulated Materials
1.3 Simulated cavity: 2 * 2
1.4 Threaded inserts must be free of oil or other contamination prior to insertion.
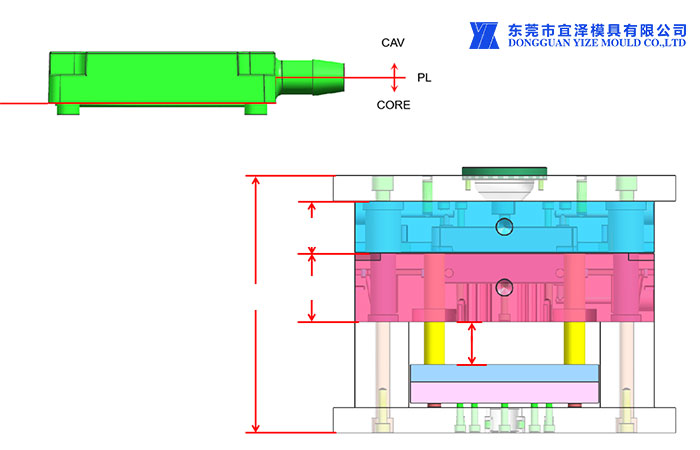
2 Parts molding allowance
2.1 Gate height: to be determined relative to the surrounding surface
2.2 The injector marks are flush to 0.13 mm below the surface.
2.3 Demoulding: need our approval
2.4 Flash Margin: 0.15mm max unless otherwise stated
2.5 Parting line mismatch: no more than 0.10 mm
2.6 Appearance: It is common to have cosmetic conditions that are an unavoidable functional design of a part or tool. In this case, the medical plastic injection mold company and the customer will agree on a control sample representing the maximum allowable conditions.
PC TM40 top and bottom cover high precision plastic injection mold machine start video:
3 Marks (customer approved character size, height, and position)
3.1 Date Code: Indicates the month and year the part was manufactured
3.2 Number of Cavities: A single mold cavity to be marked on a multi-cavity mold.

4 Finishing/Tooling
4.1 Textures: MT11020, which specifies
4.2 Polish: SPI-A2 instructed
4.3 All internal tool surfaces are 600 stone finish or equivalent quality
4.4 Mold design, including gate style and location, parting line, and ejector pin location, must be the writing of engineering before tool making.
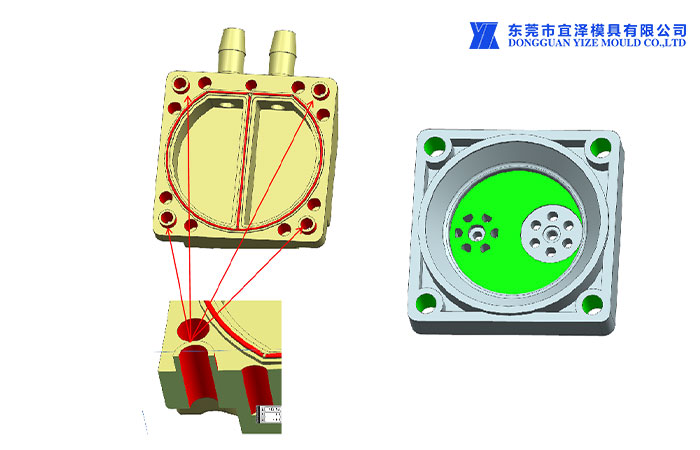
4.5 Usually only the critical dimensions that affect the fit, form and function of the part are selected.
Ensure detail is the medical plastic injection mold company's responsibility. All points in the database must remain within the linear tolerances shown in the associated tolerances

5 Packs
5.1 Parts must be adequately packaged to prevent damage during transport and handling
5.2 Identify each bag/box with part number, revision letter, manufacturer ID and quantity per bag/box.
5.3 Parts must be packaged to prevent ingress of corrugated packaging material and all other airborne contaminants Direct contact with and/or migration to parts during transport and handling

6 Medical plastic injection mold project Production Approval Requirements
6.1 First measurement: All print dimensions require at least 3 part/cavity measurements
6.2 The material will comply with the medical plastic injection mold company requirements mentioned in the applicable environment
6.3 Sample approval according to process is required prior to mass production.
6.4 SPI = Statistical Process Indicator. These dimensions will be measured at regular intervals during the molding process to verify the stability of the process. measurement is documented and maintained by the medical plastic injection mold company and available upon request.